find the electron configuration sc|Electron Configuration for Scandium (Sc, Sc3+ ion) : Tagatay After the electron configuration, the last shell of scandium has two electrons and the d-orbital has a total of an electron. Therefore, . Tingnan ang higit pa The data provided in this page was collected from University Of Caloocan City's website, other internet sources, as well as by calling or emailing the school's representatives. Please read our disclaimer. If you have found any errors or missing data, please inform us.
PH0 · What is the electronic configuration of scandium? Chemistry Ques
PH1 · Scandium Electron Configuration: 7 Easy Step
PH2 · Scandium Electron Configuration (Sc) with Orbital Diagram
PH3 · Scandium
PH4 · Find the Electron Configuration Sc
PH5 · Electron Configurations and Orbital Box Diagrams
PH6 · Electron Configuration for Scandium (Sc, Sc3+ ion)
PH7 · Electron Configuration for Scandium (Sc, Sc3+ ion)
PH8 · Electron Configuration For Scandium
PH9 · Electron Configuration Chart of All Elements (Full Chart)
PH10 · Electron Configuration Chart
PH11 · Electron Configuration Calculator
Pronunciation [edit] IPA : /si.ne.fil/ Audio: Noun [edit] cinéphile m or f by sense (plural cinéphiles) cinephile; Related terms [edit] cinéphilie; Further reading [edit]
find the electron configuration sc*******Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital. The sub-energy levels depend on the azimuthal quantum number. It is expressed by ‘l’. The value of ‘l’ . Tingnan ang higit paThe total number of electrons in scandiumis twenty-one. These electrons are arranged according to specific rules in different . Tingnan ang higit pa
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electronsof the atom revolve around the nucleus in a certain circular path. These circular . Tingnan ang higit pa
After the electron configuration, the last shell of scandium has two electrons and the d-orbital has a total of an electron. Therefore, . Tingnan ang higit pa
Atoms can jump from one orbital to another orbital in the excited state. This is called quantum jump. The ground state electron configuration of scandium is 1s2 2s2 2p6 3s2 3p6 3d1 4s2. This electron configuration shows that the last shell of the . Tingnan ang higit pa
Mar 23, 2023 Element configuration for atoms or molecules is defined by the number of electrons present in the orbit or shell. In case of Scandium, there are 3 orbits and 17 .
Scandium’s electron configuration, 1s² 2s² 2p6 3s² 3p6 3d¹ 4s², reveals how the 21 electrons are distributed among different energy levels and orbitals. Let’s delve into the .
In the case of scandium, the electron configuration is [Ar] 4s2 3d1. This means that the first two electrons fill the 1s and 2s orbitals, the next six electrons fill the .
Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. .
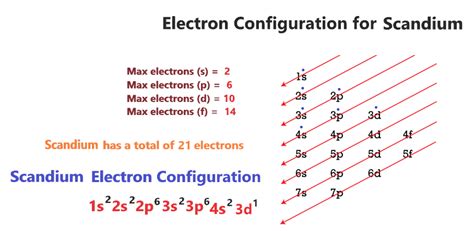
The electron configuration is the arrangement of electrons of an atom. It concerns the way electrons are distributed in the orbitals of the atom. There are 4 4 orbital types ( s s, p p, . Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or .Next, we write the electron configuration for Sc. When reading the periodic table from left to right we fill all the way to the 4s orbital and then fill a 3d orbital with the last electron. The electron configuration is 1s .Step 1: Find the electron configuration. For Cl atoms, the electron configuration is 3s 2 3p 5. Step 2: Draw the valence orbitals. Ignore the core electrons and focus on the valence electrons only. Step 3: Look for unpaired electrons. There is one unpaired electron. Step 4: Determine whether the substance is paramagnetic or diamagnetic Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of .
The electron configuration states where electrons are likely to be in an atom. If you don’t have a chart, you can still find the electron configuration. Use the element blocks of the periodic table . The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a transition metal that belongs to the d-block of the periodic table.One of the key aspects of understanding an element’s behavior and properties is its electron configuration. In this section, we will explore the electron configuration of scandium .
find the electron configuration sc Electron Configuration for Scandium (Sc, Sc3+ ion)Electronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. Electronic configuration of Scandium: The atomic symbol of Scandium is Sc. The atomic number of Scandium is 21. The atomic number of an element is equal to the number of electrons .
In several cases, the ground state electron configurations are different from those predicted by Figure 6.8.1 6.8. 1. Some of these anomalies occur as the 3 d orbitals are filled. For example, the observed ground state electron configuration of chromium is [Ar]4 s1 3 d5 rather than the predicted [Ar]4 s2 3 d4.
find the electron configuration scTo check the answer, verify that the subscripts add up to the atomic number. In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait . Principal Quantum Number (n) The principal quantum number n indicates the shell or energy level in which the electron is found. The value of n can be set between 1 to n, where n is the value of the outermost shell containing an electron. This quantum number can only be positive, non-zero, and integer values. That is, n=1,2,3,4,.. For . Electron configurations of ions. To find the electron configuration for an ion, first identify the configuration for the neutral atom. Then, add or remove electrons depending on the .

Explain why their electron configurations are different even though they have the same number of valence electrons. Answer A. Sc: [Ar] 3d 1 4s 2. Sc +: [Ar] 3d 2 *when a d-block ion forms, it pulls the 3d orbital much lower than the 4s so that all electrons that were in 4s would move to 3d. Sc +2: [Ar] 3d 1 *when a d-block ion forms, it pulls .The electron configuration of scandium is 1s2,2s2,2p6,3s2,3p6,4s2,3d1. The chemical element that belongs to the periodic table is called scandium, its atomic number is 21 and it is represented by the symbol . Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 4.3.6 4.3. 6. The valence shells of the inner transition elements consist of the ( n – 2) f, the ( n – 1) d, and the ns subshells. There are two inner transition series:1. Find the electron configuration of the following: a) silicon: [Ne] 3s 2 3p 2. b) tin: [Kr] 5s 2 4d 10 5p 2. c) lead: [Xe] 6s 2 4f 14 5d 10 6p 2. 2. Scenario: You are currently studying the element iodine and wish to use its electron distributions to aid you in your work. a) Find the electron configuration of iodine [Kr] 5s 2 4d 10 5p 5
To find the number of valence electrons for Scandium (Sc) we need to look at its electron configuration. This is necessary because Sc is a transition metal .Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first. Thus, many students find it confusing that, for example, the 5p orbitals fill immediately after the 4d, and immediately before the 6s.The filling order is based on observed experimental results, and has been confirmed by theoretical calculations.2,24,283. The electronic configuration of the first 30 elements with atomic numbers listed above corresponds to the ground state of the specific elements. Any configuration that does not correspond to the lowest energy state is called an excited state. To learn more about writing the electronic configuration of an atom or a molecule, visit BYJU .
Find 97 different ways to say DELIGHT, along with antonyms, related words, and example sentences at Thesaurus.com.
find the electron configuration sc|Electron Configuration for Scandium (Sc, Sc3+ ion)